Abstract
Galectin-3 (Gal-3) is a unique member of the galectin family of lectins. Gal-3 possesses immune-regulatory functions depending on the immune cell and the immunologic situation. There are no studies that specifically delineate the role of Gal-3 in the setting of acute GvHD but mounting research suggests that dysregulation of pathways involving the galectin family may contribute to the pathogenesis of other immune disorders. Gal-3 is expressed by many types of immune cells, including T-cells. It suppresses signaling downstream of the TCR, decreases effector T-cell cytokine production, but increases the development and differentiation of memory T cells, myeloid cells, and macrophages. We investigated the mechanisms and downstream events of Gal-3 signaling in donor T cells after Allo-HCT, using Gal-3 knockout (Gal-3 -/-) mice. We further studied the effect of Gal-3 in controlling aGvHD incidence and severity while preserving the Graft-versus Leukemia (GvL) effect by overexpressing Gal-3 in human T cells.
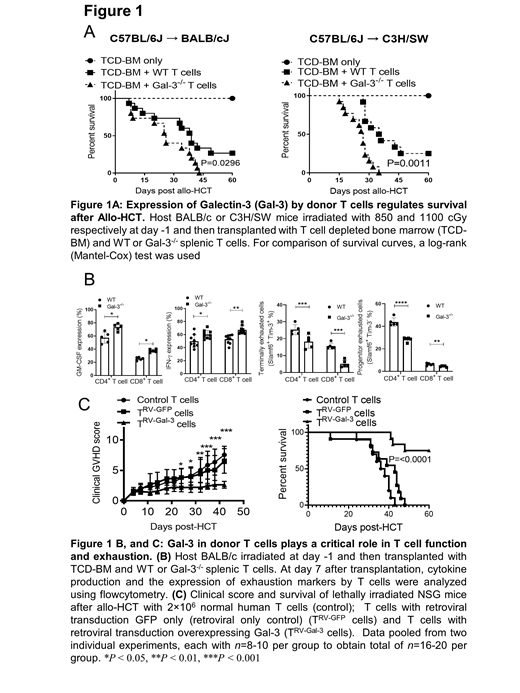
We utilized both a major MHC-mismatch (C57B/6 (H-2 b) into BALB/c (H-2 k) model and a MHC-matched, multiple minor histocompatibility antigen (miHA) mismatched B6 (H-2 b) into C3H/SW (H-2 b) model. Lethally irradiated recipient BALB/c and C3H/SW WT animals were injected with T cell depleted bone marrow alone (3 ×10 6) or with splenic T cells derived from allogeneic WT or Gal-3 -/- B6 donors (0.7 × 10 6 T cells in B6 → BALB/c and 1.5 × 10 6 in B6 → C3H/SW). We found that donor T cells express Gal-3 after Allo-HCT and that Gal-3 expression in WT T cells plays an important role in controlling GvHD, as evidenced by less severe weight loss, decreased clinical GvHD scores, and longer survival when compared to mice receiving Gal-3 -/- donor T cells (Figure 1A). We studied the mechanisms by which Gal-3 signaling controls the severity of aGvHD. Using flow cytometry analysis, we determined that Gal-3 plays a critical role in T cell proliferation and exhaustion. Gal-3 -/- T cells have a cytotoxic T phenotype with increased IFN-ℽ and GM-CSF production in T cells from the spleen and liver tissues on days 7 and 14 after Allo-HCT when compared to WT T cells (Figure 1B). There was a significant increase in T cell proliferation in Gal-3 -/- CD4 +T cells with a significantly higher level of IFN- ℽ mediated activation induced cell death (AICD) when compared to WT T cells. Gal-3 expression in T cells significantly increased the expression of exhaustion markers evidenced by a higher percentage of Slamf6 + Tim-3 + in WT T cells when compared to Gal-3 -/- T cells (Figure 1B). Gal-3 induced T cell exhaustion by through overactivation of NFAT signaling (data not shown).
We sought to determine whether overexpression of Gal-3 in human T cells could control GvHD without affecting GVL. Gal-3 was overexpressed in human T cells using retrovirus containing Gal-3, vector alone and control T cells: Gal-3 T cells (T RV-Gal-3), GFP T cells (T RV-GFP) and control T cells were injected in irradiated NSG-HLA-A2 mice. All human cells expressed HLA-A2. Gal-3 overexpression in T cells effectively controlled the severity and mortality of GvHD after Allo-HCT in this humanized murine model of GvHD, evidenced by decreased body weight loss and decreased GvHD clinical scores in recipients transplanted with Gal-3 T cells when compared to control or GFP T cells (Figure 1C). Gal-3 overexpression did not impair the GvL effect when T cells cultured with Raji and THP-1 cell lines in vitro (data not shown).
Gal-3 overexpression in T cells increased the frequencies of exhausted CD4 + T cells, and central memory CD4 + T cells while decreasing the percentage of effector CD4 T cell and INF-ℽ + CD4 + T cells. Clinical GI colon biopsies from patients undergoing allo-HCT were evaluated for Gal-3 expression in T cells using the multi-color Vectra 3 Automated Quantitative Pathology Imaging System. T cells in the colon biopsies expressed Gal-3. There was a significant correlation between Gal-3 MFI in CD4+ T cells, and GI histopathology score when analyzing Gal-3 intensity on Gal-3-expressing T cells. The Gal-3 MFI in CD4+ T cells was significantly lower in biopsies with higher colon GI histopathology scores (III-IV) compared to with lower colon GI histopathology scores I-II.
In conclusion, these data reveal how Gal-3 can influence donor T cell proliferation and function in preclinical aGvHD models and point to the feasibility of manipulation of Gal-3 signaling to ameliorate aGvHD in the clinical setting.
Blazar: Rheos Medicines: Research Funding; Carisma Therapeutics, Inc: Research Funding; Equilibre Pharmaceuticals Corp: Research Funding; Tmunity Therapeutics: Other: Co-founder; BlueRock Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Magenta Therapeutics: Membership on an entity's Board of Directors or advisory committees. McCarthy: Magenta Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bluebird: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Juno: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal